39 fda health claims on food labels
health claims and food labels Flashcards - Quizlet GRAS, EAFUS, nutrition labeling and education act, DSHEA, food and drug modernization act, public health security and bioterrorism preparedness response act GRAS generally recognized as safe. dont need a formal premarket review if added to foods. safety established by long history of food. know nature, intended use, and safety of product. The Basics of Food Product Health Claims - LabelCalc For additional information from the FDA on Health Claims, Download this PDF and scroll to Appendix C. Claim #3: Qualified Health Claims. Qualified food product health claims refer to a claim made on a food product label in reference to a disease that does not have to meet the rigorous standards of an Authorized Health Claim.
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified.". Authorized Health Claims: Claims that have significant scientific agreement (SSA).

Fda health claims on food labels
Food Label Claims: What You Can and Can't Trust - WebMD The FDA is updating its definition for this claim. Until then, companies can make the "healthy" claim if the fats in their foods are mostly mono- and polyunsaturated fats. The healthy claim also... Qualified Health Claims - FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...
Fda health claims on food labels. Introduction to Food Product Claims - FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". Qualified Health Claims - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions. Health Claims - Canada Health claims are also subject to Section 3 of the Food and Drugs Act that prohibits the labelling and advertising of any food to the general public, as a treatment, preventative or cure for any diseases and health conditions listed in Schedule A of the Food and Drugs Act. What are some examples of an FDA health claim on a food label? Health Claims - Require premarket approval by the U.S. Food and Drug Administration (FDA) if they are intended for use on the label of foods or dietary supplements. Qualified health claims are based on less scientific evidence than authorized health claims and require disclaimers or qualified wording.
FDA perspectives on health claims for food labels - PubMed FDA perspectives on health claims for food labels Abstract The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA). PDF "GMO-Free" Claims and False and Misleading Food Labels—Why ... articles of food, or articles which enter into the composition of food, the package or label of which shall bear any statement, design, or device regarding such article, or the ingredients or substances contained therein which shall be false or misleading in any particular… --Pure Food and Drug Act of 1906 (P.L. 59-384, 34 Stat. 768). 12 ABC's of Health Claims - WebMD The FDA's proposed Consumer Health Information for Better Nutrition program wants to improve your understanding of the health consequences of your food choices. Their plan is to tighten ... Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... Authorized Health Claims That Meet the Significant ... Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food... Definitions - Health claims on food labels - Food label ... Health claim Any representation in labelling or advertising that states, suggests, or implies that a relationship exists between the consumption of a food and health. Laxation The normal softness and bulking of the stool resulting from such factors as increased undigested residue or bacterial mass, trapping of gases or water retention. CFR - Food and Drug Administration (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements...
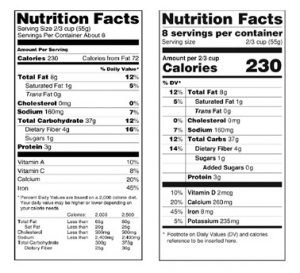
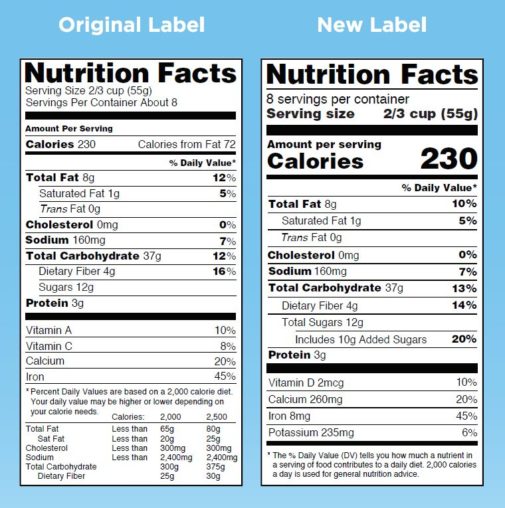
Understanding Food Labels | The Nutrition Source | Harvard ... The FDA has approved 12 health claims on food labels such as the relationship between calcium and osteoporosis; sodium and hypertension; fiber-containing grains, fruits and vegetables and cancer; and folic acid and neural tube defects. However, just because a food contains a specific nutrient that is associated with a decreased risk of disease ...
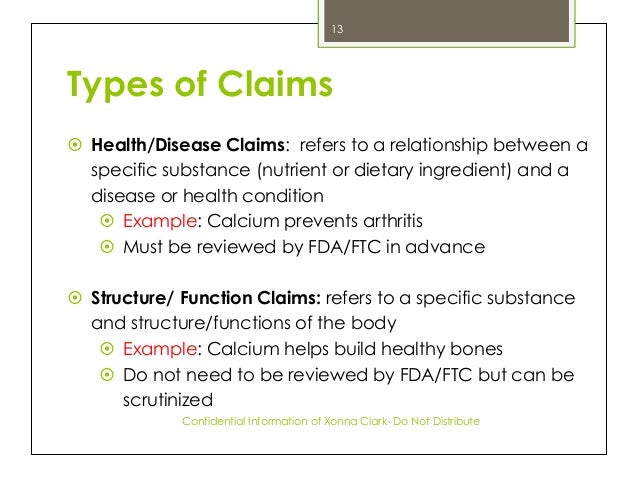
Food Packaging Claims - American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims.
Food Labeling - USDA The U.S. Food and Drug Administration (FDA) with the U.S. Department of Agriculture (USDA) and the Environmental Protection Agency (EPA) initiative to provide consumers with science-based educational information to better understand how GMOs are made, learn more about the types of crops that have been modified, address questions they may have about the health and safety of GMOs as well as ...
21 CFR - LII / Legal Information Institute (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements (e.g., a brand name including a term such as "heart"), symbols (e.g., a heart symbol), or vignettes, characterizes the relationship of any substance …
Health claim - Wikipedia A health claim on a food label and in food marketing is a claim by a manufacturer of food products that their food will reduce the risk of developing a disease or condition. For example, it is claimed by the manufacturers of oat cereals that oat bran can reduce cholesterol, which will lower the chances of developing serious heart conditions.
What You Need to Know About Health Claims on Food Labels ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims)
The FDA has approved health claims on food labels for all ... What was the holding by the US Supreme Court? Model health claims approved by the FDA cannot be modified or abbreviated when placed on food labels "Adequate calcium and vitamin D along with regular exercise may reduce the risk of osteoporosis" is an FDA approved health claim and can be used on food labels.
5 Understanding Food Labels and Health Claims - Maricopa Health Claims & Foods To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease."
Drugs vs. foods - Health claims on food labels - Food ... See Acceptable Disease Risk Reduction Claims and Therapeutic Claims for acceptable examples. Furthermore, Section 3, FDA, prohibits the sale of a food that is labelled or advertised to the general public as a treatment, preventative or cure for any of the diseases referred to in Schedule A. Previous Table of Contents Next
ABC's of Health Claims - WebMD Sorting through the health claims plastered on products is like walking a minefield. The good news is that the FDA promises to enhance enforcement of the new rules and regulations, which should ...
Health Claims - University of Texas at Austin According to the United States Food and Drug Administration (FDA) there are only three categories of claims that are approved to be printed on food packaging: health claims, nutrient claims, and function claims. Generally, these labels are found on the front side of the food package in emphasized lettering. Health Claims
FDA sued over health claims on food labels | Center for ... CSPI legal affairs director Bruce Silverglade warns that the FDA's new system will mislead consumers when sketchy health claims begin appearing on food labels. "Food manufacturers could begin claiming that chocolate may reduce the risk of heart disease or that ketchup may reduce the risk of cervical cancer," Silverglade said.
Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...
Qualified Health Claims - FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".









Post a Comment for "39 fda health claims on food labels"